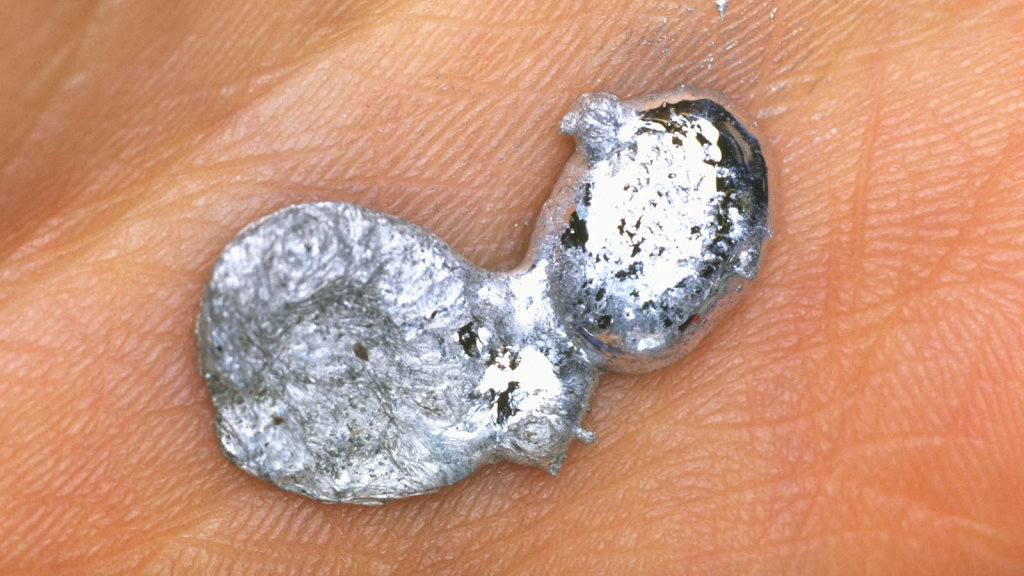
Gallium is a rare, silvery white element that can pull off one of the coolest parlor tricks on the periodic table. At room temperature, gallium is a shiny metallic solid that resembles pure aluminum. But hold it in your hands for a few minutes and this solid hunk of metal starts to melt.
Yup, the melting point of gallium is just 85.6 degrees F (29.8 degrees C), which means that it melts into a mirror-like puddle in your hot little hand. In its liquid form, gallium looks a lot like mercury, but gallium isn’t toxic like mercury so it’s safer to handle (although it can stain your skin).
But gallium is so much more than fodder for melt-in-your-hand YouTube videos. It’s also a key ingredient in LED lights and the go-to semiconductor material for the powerful microchips in your smartphone. The only thing stopping gallium from taking over the electronics world is that it’s very rare and very expensive compared to silicon.
Mendeleev Predicted the Existence of Gallium
Contents
Pure gallium doesn’t exist in its shiny elemental form in nature. It needs to be extracted from minerals like bauxite through a multi-step chemical process. According to the U.S. Geological Survey, the abundance of gallium in Earth’s crust is a measly 19 parts per million (silicon, by comparison, is 282,000 parts per million). The first person to isolate and recognize gallium as a new element was the French chemist Paul-Emile Lecoq de Boisbaudran in 1875. He named it gallium after the Latin name for France, “Gallia.”
But four years before Boisbaudran’s discovery, the famed Russian chemist Dmitri Mendeleev predicted gallium’s existence. Mendeleev, known as the “father of the periodic table,” saw that there was a gap in the table after aluminum, so he posited that a missing element he called “eka-aluminum” would share many of the properties of aluminum, but with a different atomic structure.
Mendeleev was right, but he couldn’t have predicted how gallium’s unusual qualities — somewhere between a metal and a nonmetal — would make it ideal for modern electronics.
Advertisement
An Element With an Identity Crisis
Here’s another cool and somewhat bizarre fact about gallium: While it melts at just 85.6 degrees F (29.8 degrees C), it doesn’t boil until a scorching 3,999 degrees F (2,204 degrees C). That earns gallium the award for maintaining the longest liquid phase of any element. But why does that happen?
“Gallium is confused,” says Daniel Mindiola, a chemistry professor at the University of Pennsylvania who we reached through the American Chemical Society. “It melts at a low temperature, which is consistent with a light element, but it boils at a very high temperature, which is consistent with a very heavy element. Gallium doesn’t know if it wants to be a metal or a nonmetal.”
Gallium’s dual personality stems from where it sits on the periodic table among two groups called the “metalloids” and the “post-transition metals.” Gallium is next in line after aluminum, but its atoms are far more “independent” than its shiny foil (get it?) and aluminum is more “electropositive,” says Mindiola, a trait of true metals.
Like silicon, gallium is a good conductor of electricity, but not a great one. That’s what makes both of these metalloids prime candidates for semiconductors, where the flow of electricity needs to be controlled.
“Gallium is actually the ideal semiconducting material, even better than silicon,” says Mindiola. “The problem is it’s rare, so it’s expensive.”
Using current manufacturing processes, a wafer of gallium arsenide, the most popular gallium-based semiconductor material, is roughly 1,000 times more expensive than a silicon wafer.
Advertisement
There’s Gallium in Your Gadgets
Even though gallium is much more expensive than silicon, it’s become a popular semiconductor material in the latest generations of smartphones. Smartphones communicate with cellular data networks using radio frequency (RF) chips, and RF chips made with gallium arsenide give off less heat than silicon and can operate at higher frequency bands, a requirement for 5G networks. A little more than 70 percent of all the gallium consumed in the U.S. is used to make RF chips and other types of integrated circuits, according to the USGS.
But one of the coolest applications of gallium is in light-emitting diodes (LEDs), which are now used in everything from computer displays to traffic lights to luxury car headlights. LEDs are so popular because they are super-efficient, converting electricity directly into light. The first visible-light LEDs were invented in the early 1960s when researchers at General Electric discovered the unique properties of diodes made with various gallium alloys (combinations of gallium, arsenic, nitrogen, phosphorus and other elements).
In a diode, electrons move through two layers of semiconductor material, one with a positive charge and the other with a negative. As free electrons from the negative side fill “holes” in the positive side, they emit a photon of light as a byproduct. Scientists have discovered that different gallium alloys emit photons of different visible light frequencies. Gallium arsenide and gallium phosphide produce red, orange and yellow light, while gallium nitride produces blue light.
“Just apply a current to an LED and it lights up like a Christmas tree,” says Mindiola.
Not only do LEDs produce light when connected to electricity, but the process can be reversed. The special diodes inside of solar cells are also made of gallium-based semiconductors. They take incoming light and separate it into free electrons and “holes,” generating voltage that can be saved in a battery as electricity.
Advertisement
Other Nifty Uses of Gallium
“Medicine is beginning to use gallium, too, for detecting and treating certain types of cancers,” says Mindiola. “Gallium-67 is attracted to cells that replicate faster than normal, which is what happens in a tumor.”
Gallium-67 is a radioactive isotope of gallium that emits non-toxic gamma rays. Radiologists can scan a patient’s whole body for tumors or inflammation from an infection by injecting gallium-67 into their bloodstream. Since gallium-67 binds to clumps of fast-growing cells, those potential trouble spots will show up on a PET scan or any other scan that’s sensitive to gamma rays. Gallium nitrate has also shown effectiveness in shrinking and killing certain types of tumors, not just detecting them.
The aerospace industry has been hot on gallium for decades. All the high-end solar panels that power satellites and long-range spacecraft are made with gallium arsenide, including the critical solar panels on the Mars Exploration Rovers. At peak performance, the gallium-based solar cells on the Mars rovers could produce 900 watt-hours of energy per Martian day.